Contact us:
Dr. Zifei Liu

Assistant Professor
Biological & Agricultural Engineering
Kansas State University
043 Seaton Hall
Manhattan, KS 66506
Email: Zifeiliu@ksu.edu
Phone: 785-532-3587
Fax: 785-532-5825
Research

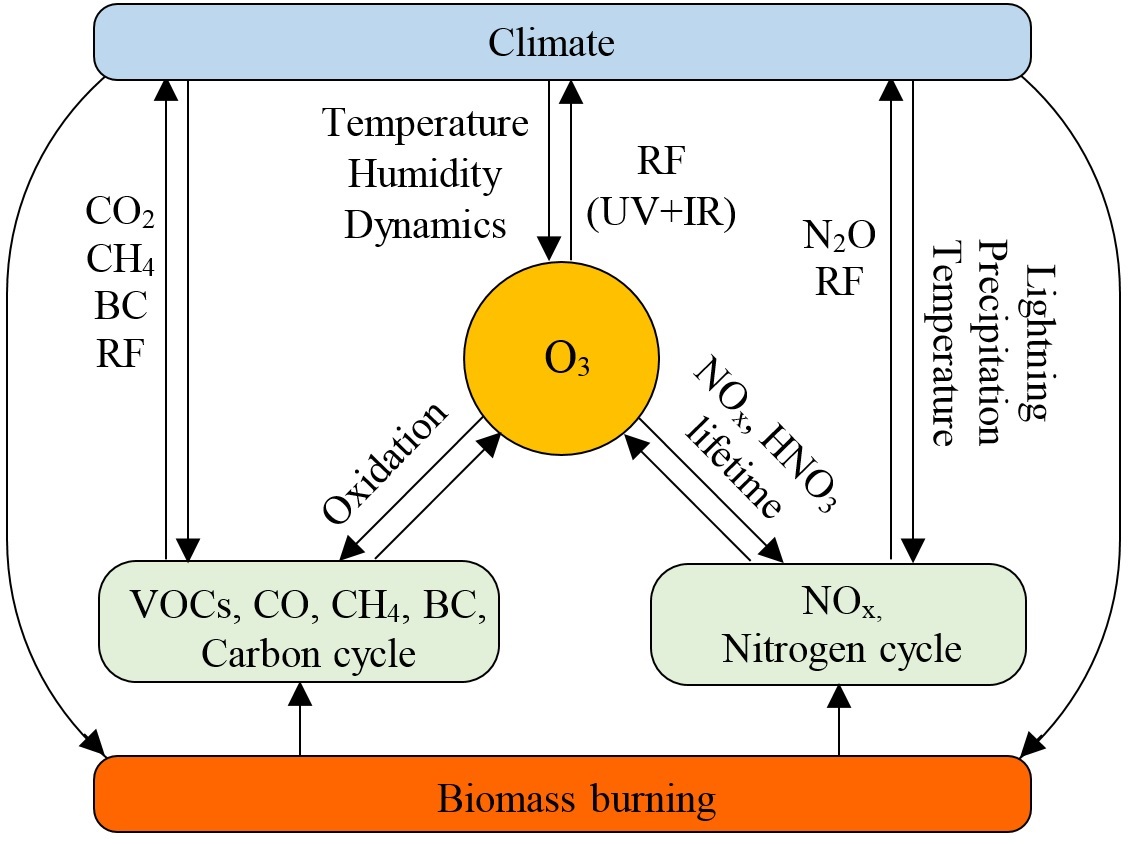
Interaction of fire, atmospheric composition and climate in the earth system
Fire is a worldwide phenomenon and a central component of the earth system. Biomass burning links the biosphere and the atmosphere via release of smoke, with implications for air quality, human health, climate change, and the economy. Smoke from fires is a complex mixture of gases and aerosols including hundreds of species. CO2, CO, NOx and VOCs are the most important emitted gases, while black carbon (BC) and organic carbon (OC) are the most important aerosol species. Interaction of fire, atmospheric composition, and climate in the earth system is illustrated in the following figure.
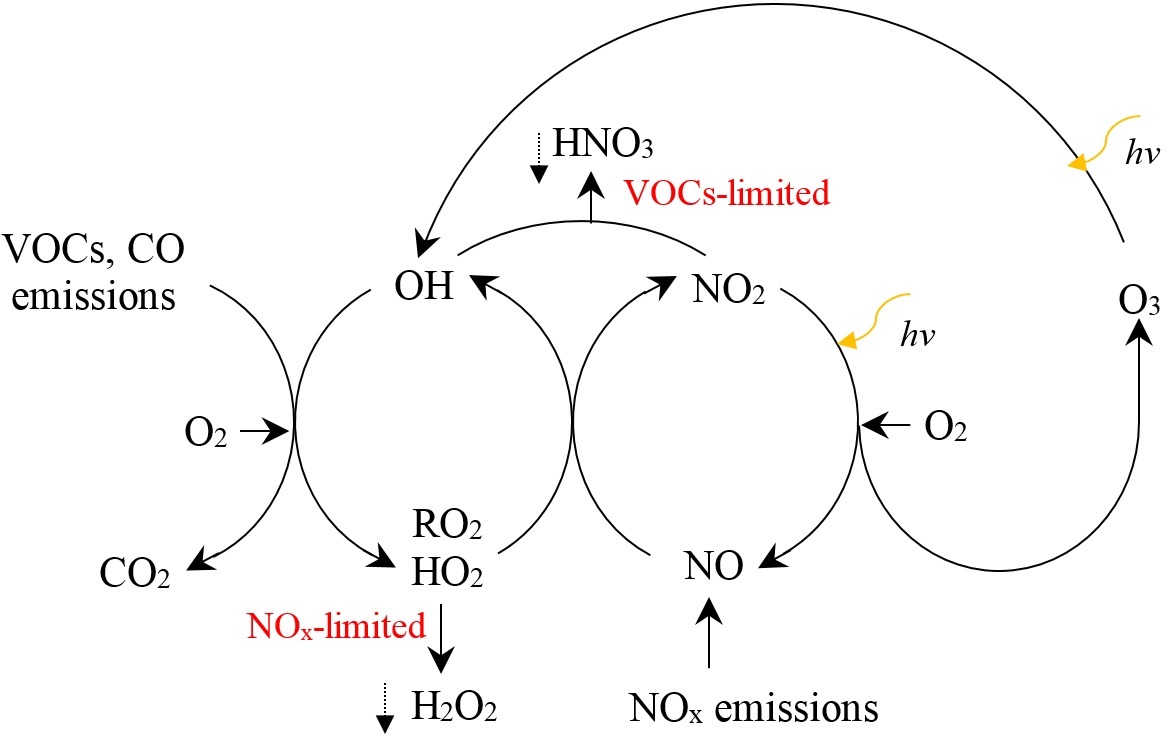
Central role of O3 in atmospheric oxidation chemistry and climate change
O3 is a reactive oxidant that is formed in the troposphere by photochemical reactions of volatile organic compounds (VOCs) and CO which are catalyzed by nitrogen oxides (NOx). O3 production is initiated by reactions that generate hydroxyl (OH) and hydroperoxy (HO2) radicals, and organic peroxy (RO2). OH oxidizes VOCs and other precursors to form RO2 and HO2, which convert nitrogen monoxide (NO) to NO2. NO2 photolyzes to form an oxygen atom, which combines with molecular oxygen to form O3. A simplified scheme is shown in the following figure. Dependence of O3 production on its precursors is nonlinear. At NOx-limited region, O3 production increases with NOx and is insensitive to VOCs. At high NOx levels, O3 production increases with VOCs (VOCs-limited region), but may decrease as NOx increases because of increasingly effective HO removal due to HO-NO2 reactions. For much of the troposphere, O3 production is typically limited by NOx and methane (CH4) due to sufficient natural sources of VOCs. Local O3 production within the planetary boundary layer (PBL) is sometimes inefficient due to high NOx levels. However, transportation of emitted NOx can lead to disproportionately large O3 production downwind or in the free troposphere. Although NOx has a lifetime of only a few days, peroxyacetyl nitrate (PAN) may serve as reservoir for NOx, and be transported long distances, and then result in NOx release and O3 production in the remote troposphere. CO is a product of incomplete combustion, with an atmospheric lifetime of about two months. It is a precursor of O3, and a long-lived tracer of BB influence. As most VOCs react with OH and are subsequently oxidized to HCHO, HCHO can serve as a proxy for total VOCs chemical reactivity with OH, and it may also serve as an indicator of secondary organic aerosol (SOA) formation. The variability in the distribution of HCHO is highly correlated with isoprene. Valuable information on O3 distribution and chemical sensitivity of O3 production could be inferred through combined analysis of NOx, CO and HCHO data.
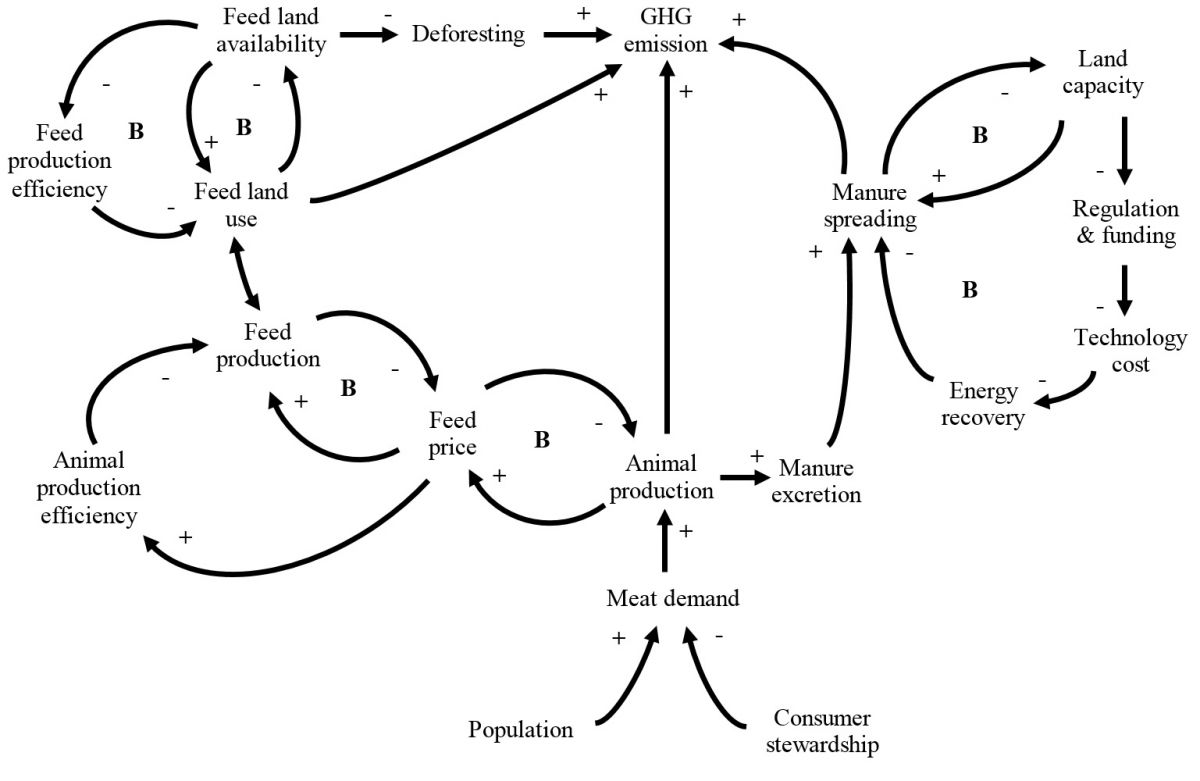
Mitigation of Greenhouse Gas Emissions from Animal Production
Accurate quantification of the carbon footprint of animal products and developing greenhouse gases (GHG) mitigation strategies accordingly are of interest for consumers, the general public, as well as the academic community. The objective of this review was to summarize recent advances in GHG emission quantifications, life cycle assessment applications, as well as mitigation technologies for animal production in the United States., in order to assist the development of system-based solutions for mitigation of GHG emissions from animal production. The GHG emissions from animal production mainly come from feed production, enteric fermentation, and manure management. Opportunities to mitigate emissions from feed production largely rely on continuous improvements in animal and feed production efficiency. This is in general agreement with the economic interest of the industry. To mitigate emissions from manure, many technologies that can be chosen, depending on the given economic and regulatory environments. It is possible to minimize GHG emissions from manure through manure energy recovery when economic feasibility is achieved. For enteric emissions, there are limited opportunities to reduce GHG emissions through dietary manipulation, feed management, or feed supplementations. Improving environmental stewardship of consumers, reducing food waste, will reduce animal protein demand and is an important bottom-line strategy to mitigate GHG from animal production systems.
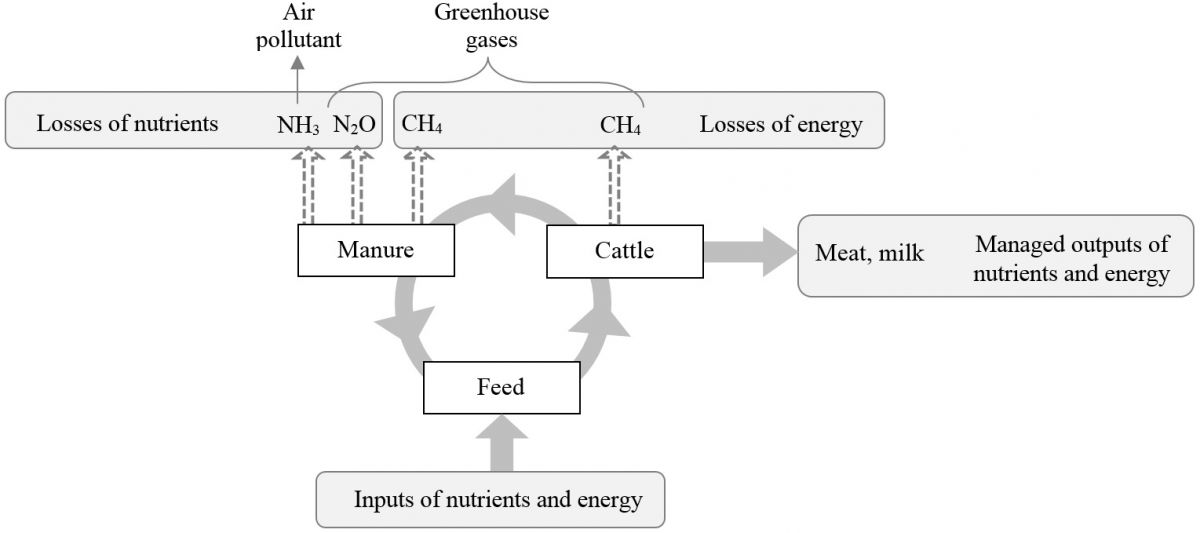
Ammonia and Methane Emissions Expressed as Losses of Dietary Nutrients or Energy
With increasingly stringent air pollution regulations and the emerging pressure to regulate agricultural enterprises, there will be a continued need for emission inventories based on reliable and representative emission factors, as well as an increasing demand for mitigation strategies. On the other hand, production efficiency is a contributing factor in reducing the environmental footprint per unit of product. Strategies that increase production efficiency will conserve resources and improve environmental stewardship, and could represent a great opportunity for mitigating NH3 and CH4 emissions per unit of livestock product. In evaluating these mitigation practices, quantifying NH3 and CH4 emissions as a percentage of losses of dietary nutrients or energy would be beneficial. In addition, emission factors based on intakes of dietary nutrients or energy can address the effect of feed intake and reduce uncertainties in estimation of emissions, and also be used as a key parameter in establishing emission inventories. Read more ......
Improving estimation of enteric methane conversion factors for beef cattle
Enteric methane (CH4) from beef cattle is an important greenhouse gas, and it represents an unproductive loss of dietary energy. The CH4 conversion factor (Ym), which is defined as the percentage of general energy intake converted to CH4, is a key parameter in determining and modeling CH4 emissions from beef cattle. The Ym value could be affected by various factors including feed properties and animal attributes. These effects have not been sufficiently documented. Reported Ym in literature had wide variations, ranging from 2% to 11.5%. The objectives of this study was to conduct a systematic literature review in order to identify the sources of variations in reported Ym values for beef cattle through a meta-analytical approach. Specifically, an exhaustive search was conducted to collect all available data in literature, including the Ym value and all corresponding feed properties and animal attributes when the specific Ym value was measured. Statistical analyses were conducted to investigate how feed digestibility, intake level, breed of cattle, geographic region and other factors may affect the Ym value. Results of this study improved estimation of CH4 emissions from beef cattle according to various animal husbandry practices, and may provide new thoughts on CH4 mitigation strategies. Read more ......
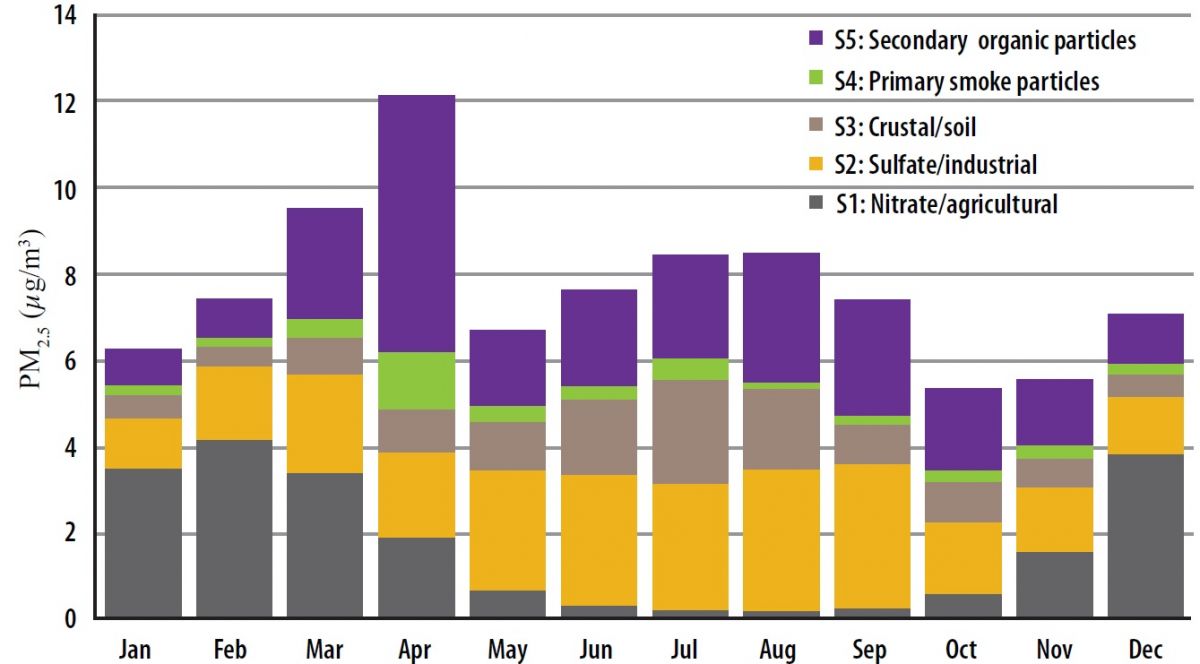
Estimate contributions of prescribed rangeland burning in Kansas to ambient PM2.5 through source apportionment with Unmix receptor model
The US EPA Unmix6.0 receptor model was applied to the speciated PM2.5 data from 2002 to 2014 (n = 1428) at the Tallgrass site. The modeling identified the following five source categories (S1 to S5) that contribute to local ambient PM2.5: S1-nitrate/agricultural (22%), S2-sulfate/industrial (30%), S3-crustal/soil (14%), S4-primary smoke particles (5%), and S5-secondary organic particles (29%). S4 includes mostly primary smoke particles from burning. S5 is composed of secondary organic particles which are particulates formed from atmospheric organic species through gas-to-particles transformation. S5 can be associated with biogenic, mobile or smoke emissions, such as those from rangeland burning. Both S4 and S5 were heavily affected by episodic smoke emissions from rangeland burning and had consistent spikes in April. The monthly average contributions of S4 and S5 to PM2.5 were 11% and 49% in April as compared with annual averages of 5% and 29% respectively. In April, around two-thirds of the secondary organic particles (S5) at the Tallgrass site could be attributed to rangeland burning. The average contribution of secondary organic particles from smoke was four times higher than that of primary smoke particles. Read more......
A review of practices and technologies for odor control in swine production facilities
Odors from swine facilities comprise hundreds of chemicals, including volatile organic compounds (VOC), ammonia (NH3), and hydrogen sulfide (H2S). Facility maintenance and management practices to reduce impact of odor are reviewed in regard to regular cleaning of facilities, ventilation, floor design, drainage and manure removal systems, frequent manure removal, manure storage, and odor separation distances. Approaches to control odor and air pollution can be classified into three categories: ration/diet modification, manure treatment, capture/treatment of emitted gases and enhanced dispersion. Each of these mitigation approaches includes several specific technologies, which are summarized in tables with an evaluation of overall cost and brief comments on advantages or limitations of each technology. Diet modification strategies have been shown to reduce NH3 emissions effectively with low cost and should be considered as a best management practices, although their effectiveness in reducing odor is still uncertain. Permeable covers and biofilters seem to have great potential to be the most promising and cost effective technologies for manure storage facilities and swine houses respectively. However, both of the technologies need careful maintenance to perform effectively. Care must be taken to select technologies that are compatible with the management capabilities of the operation to prevent potential failure due to mismanagement. Read more ......
Co-digestion of Dairy Manure
Anaerobic digestion (AD) is a proven waste treatment technology for producing biogas as renewable energy and reducing greenhouse gas and odor emissions. However, the economics of treating animal manure using AD are not always favorable due partly to the low biogas yield. One approach for increasing biogas yield is to co-digest manure with more degradable wastes. Co-digestion of dairy manure and glycerine (a biodiesel by-product) with ultrasonic pre-treatment was recently studied to enhance biogas production. Up to 800% increase in biogas production has been reported comparing to untreated manure only digestion. However, the mechanisms behind the processes are still not fully understood and an optimization of the technology is needed.
Photocatalytic Technology Using UV/TiO2
Titanium dioxide (TiO2) has been widely used as photocatalyst. When a TiO2- treated surface is irradiated with UV, an electron-hole pair is created and the hole generates highly reactive hydroxyl radicals, which can oxidize and break down many organic and inorganic air pollutants.The UV/TiO2 was recently studied for treating livestock emissions and demonstrated great prospects. Livestock odor can be mitigated into less odorous or odorless products and the process destroys bacteria and viruses.
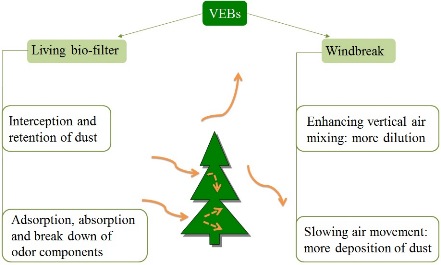
Vegetative Environmental Buffers (VEBs)
VEBs are potential cost effective strategy for reducing dust, odor, NH3 and H2S from farms, although effectiveness and costs are highly variable and depend on site specific design.
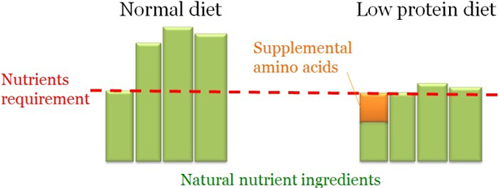
Dietary Strategies
Diet usually supply far more protein than is required to satisfy the requirement for the most limiting nutrient. Low protein diets can be used when supplemental amino acids are formulated to provide the limiting nutrient in the diet. Up to 36% reduction in NH3 was observed using low protein diet in livestock production.
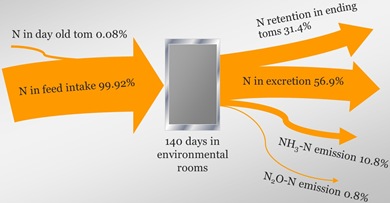
Nitrogen Mass Balance and Flow
The NRC recommended that process based models should be used with mass balance constraints for nitrogen-containing compounds. The following is nitrogen balance from a turkey tom study.
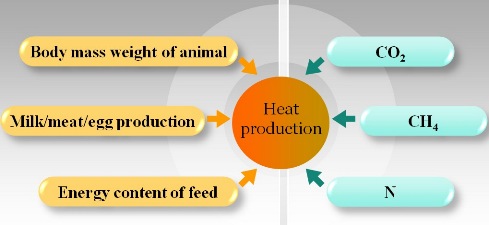
Modeling CO2 Production of Animals
Heat production of animals can be determined from body weights and production levels. It can also be related with O2 consumption, CO2 production, CH4 production, and nitrogen excretion of the animal. The CO2 productin of animals can be modeled through these relationship.
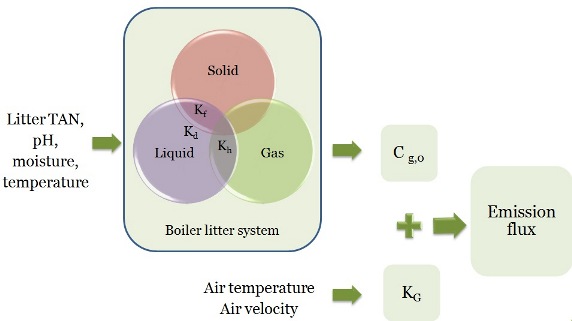
Ammonia Emission Model
The emission flux is function of an equilibrium concentration Cg,0 and a mass transfer coefficient Kg while the equilibrium concentration can be estimated from litter properties using a sub-model that simulate the three phase litter system, and Kg can be estimated from air temperature and air velocity.
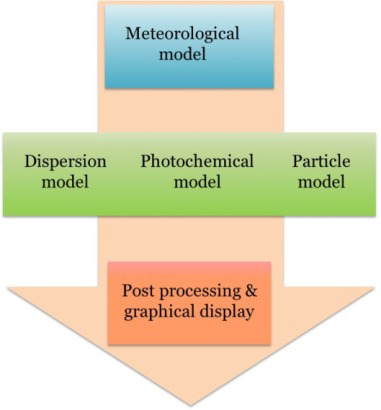
Air Quality Model
Air quality model is usually a complex system including meteorological model, dispersion model, photo-chemical model, and particle model. AERMOD and CALPUFF are two regulatory air quality models that are widely used in industry. Efforts are needed to study how these models can be applied for agricultural sources.